High school level knowledge of crystal lattices
2023/10/01
- 1 iOS app ‘Crystal Lattice’
- 2 How to use ‘Crystal Lattice’
- 3 Download ‘Crystal Lattice’
- 4 Other apps
iOS app ‘Crystal Lattice’
The iOS app ‘Crystal Lattice’ is an app for learning the basics of crystal lattices.
The content is generally high school level.
It’s a free app. (∴ Banner ads are displayed on the top page.)
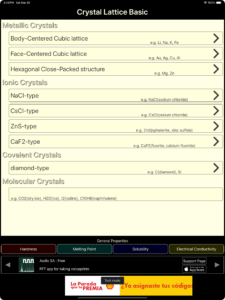

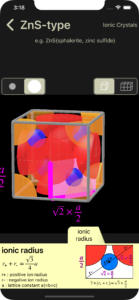

The iOS app ‘Crystal Lattice’ contains basic knowledge (university entrance exam level) of crystal lattices.
When learning about crystal lattices, you may find it difficult to understand the 3D structure, and this app will come in handy at such times.
The iOS app ‘Crystal Lattice’ allows you to scale and rotate the 3D displayed crystal lattice. Promote 3D understanding by looking at things from various angles.
For metal crystals and covalent crystals, this app explains how to calculate the coordination number, number of atoms in the lattice, atomic radius, and atomic packing factor.
For ionic crystals, this app explains how to calculate the coordination number, number of ions in the lattice, ionic radius, and limiting radius ratio.
Regarding metal crystals, this app explains body-centered cubic lattice, face-centered cubic lattice, and hexagonal close-packed structure.
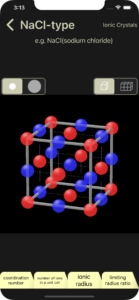
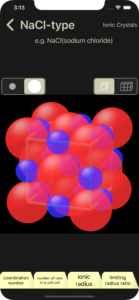
Regarding ionic crystals, this app explains NaCl type, CsCl type, ZnS (zincblende) type, and CaF2 (fluorite) type.
Regarding covalent crystals, this app explains Diamond type.
How to use ‘Crystal Lattice’
The iOS app ‘Crystal Lattice’ is easy to use. On the startup screen, simply open a learning title such as ‘Body-Centered Cubic Lattice’ and select a learning item from the tabs that appear at the bottom.
The crystal lattice can be scaled and rotated at any time. In addition, the following switching is also possible.
・Switching the display size of atoms (display center point/display atomic radius)
・Switching the number of atoms to display (display atoms in one unit lattice/display atoms in multiple unit lattices)
Open learning title (3D screen)
From the learning titles lined up in the center of the startup screen, tap the right angle bracket button to open the 3D screen.
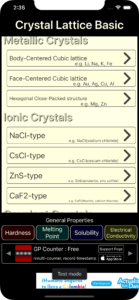
The learning titles that can be opened are as follows. (There are no learning titles that can be opened for the molecular crystals.)
・Body-Centered Cubic lattice (Metallic Crystals)
・Face-Centered Cubic lattice (Metallic Crystals)
・Hexagonal Close‐Packed structure (Metallic Crystals)
・NaCl-type (Ionic Crystals)
・CsCl-type (Ionic Crystals)
・ZnS-type (Ionic Crystals)
・CaF2-type (Ionic Crystals)
・diamond-type (Covalent Crystals)
3D screen: Basic operations
Switching the display size of atoms
You can change the display size of atoms/ions at any time using the selector at the top left.
・Switching the number of atoms to display (display atoms in one unit lattice/display atoms in multiple unit lattices)
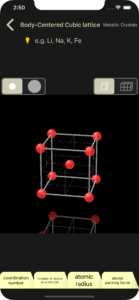
display center point
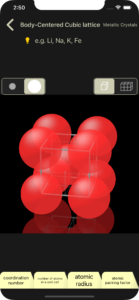
display atomic radius
Switching the number of atoms to display
You can change the number of atoms/ions at any time using the selector on the top right.

display atoms in one unit lattice

display atoms in multiple unit lattices
Scale, Rotate, Reset
You can zoom in or out the crystal lattice at any time by pinching in/out.
You can rotate the crystal lattice at any time by dragging it. (There is a limit to the size of the rotation angle, and it will not rotate beyond that limit.)
Double-clicking on the screen will reset the scaling and rotation to the initial state.
Learning items (metallic crystals, covalent crystals)
We will explain the learning items (coordination number, number of atoms in a unit cell, atomic radius, atomic packing factor) using a body-centered cubic lattice as an example.
When you tap the tab of the target learning item at the bottom of the screen, related diagrams, formulas, etc. will be displayed. Tap the tab again or swipe down on the displayed area to make it disappear.
When displaying, the 3D image will be automatically changed as necessary.
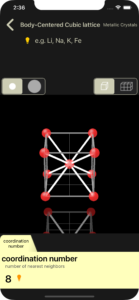
coordination number
One atom and the atoms that touch it are connected by white lines. The number of white lines is the coordination number.
There is an orange light bulb button to the right of the displayed coordination number. Tapping this will perform a white line display animation.
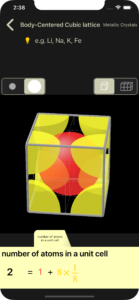
number of atoms in a unit cell
For each atom, the part that protrudes from the unit lattice is cut and displayed.
Atoms are displayed in different colors depending on the amount contained in the unit cell (in the example of a body-centered cubic lattice below, 1 atom is red and 1/8 atom is yellow). Corresponding to this color, the calculation formula for the number of atoms in a unit cell is also displayed in different colors.
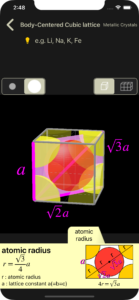
atomic radius
For each atom, the part that protrudes from the unit lattice is cut and displayed.
The cut plane to be focused on to calculate the atomic radius is displayed in pink. Some atoms are displayed fainter to make it easier to see the cut plane.
“the length of the diagonal of a square with side length a is √2a” and “the length of the diagonal of a cube with side length a is √3a” are known. Calculations are omitted.
atomic packing factor
Displays the formula for calculating the atomic packing factor using the atomic radius and number of atoms in a unit cell calculated in other learning items.
Calculation results are displayed with decimal places rounded down, such as 68%.

Learning items (ionic crystals)
The learning items (coordination number, number of ions in a unit cell, ionic radius, limiting radius ratio) will be explained using the NaCl-type as an example.
When you tap the tab of the target learning item at the bottom of the screen, related diagrams, formulas, etc. will be displayed. Tap the tab again or swipe down on the displayed area to make it disappear.
When displaying, the 3D image will be automatically changed as necessary.
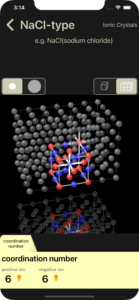
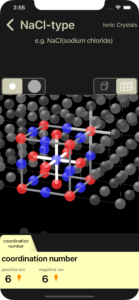
coordination number
The coordination number of positive ion and negative ion are displayed. In the 3D image, positive ions are shown in blue and negative ions are shown in red.
A white line connects one positive ion and the negative ions that are in contact with it. The number of white lines is the coordination number of positive ion.
A slightly darker white line connects one negative ion and the positive ions that are in contact with it. The number of slightly darker white lines is the coordination number of negative ion.
There is an orange light bulb button to the right of the displayed coordination number. Tapping this will perform a line display animation.
number of ions in a unit cell
For each ion, the portion that protrudes from the unit cell is cut and displayed.
Ions are displayed in different colors depending on the amount contained in the unit cell. Positive ions are color-coded with colors closer to blue, and negative ions are color-coded with colors closer to red. Corresponding to this color, the formula for calculating the number of ions in a unit cell is also displayed in different colors.

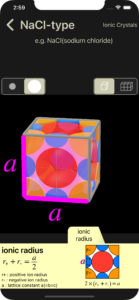
ionic radius
For each ion, the portion that protrudes from the unit cell is cut and displayed.
The cut plane of interest for calculating the ionic radius is displayed in pink. For some ions, the display transparency may be increased to make it easier to see the cut plane. (In the example below, there are no ions that increase transparency)
“the length of the diagonal of a square with side length a is √2a” and “the length of the diagonal of a cube with side length a is √3a” are known. Calculations are omitted. (These are not used in the example below.)
limiting radius ratio
The limiting radius ratio is the ionic radius ratio (r+/r-) when negative ions touch each other. (Negative ions are usually larger than positive ions)
The radius ratio (r+/r-) of the cross section for calculation is limitng radius ratio. Similarly, ions in 3D images are also displayed with a radius of limiting radius ratio.
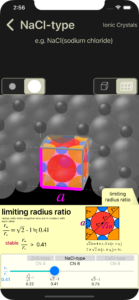
You can change the ion radius ratio (r+/r-) of cross-sectional views and 3D images using the slider. While sliding (while touching your finger), you can set the radius ratio to less than the limiting radius ratio, but after sliding (when you release your finger), the radius ratio returns to the possible range.
In the above example of the NaCl-type (coordination number 6), the limiting radius ratio is 0.41, but if you try to set a radius ratio lower than that, for example 0.3, negative ions will overlap. (a situation that normally cannot occur) (Displayed only while your finger is touching)
Changing the ion radius ratio (r+/r-) is only possible for 1:1 positive ion/negative ion types. In other words, it can be changed for NaCl-type (coordination number 6), CsCl-type (coordination number 8), and ZnS-type (coordination number 4). It cannot be changed for CaF2-type. (For CaF2-type, there is no learning item tag for limiting radius ratio.)
Deepen your understanding of the close-packed structure of face-centered cubic lattices
Face-centered cubic lattice and hexagonal close-packed structure are both close-packed structures with an atomic packing factor of 74% and are similar, but as long as you look at the unit lattice, they are completely different and you can’t believe that they are similar.
In order to compare the close-packed structure of a face-centered cubic lattice with that of a hexagonal close-packed structure, we recommend that you look at the face-centered cubic lattice from the upper right front.

The first layer from the front of the screen shows one atom, the second layer shows six atoms, and the third layer shows 15 atoms.
This corresponds to the direction when looking at the hexagonal close-packed structure from directly above. It is so similar to the hexagonal close-packed structure that you can hardly tell the difference.
Now, if you erase the six atoms in the second layer, we can see the difference from the hexagonal close-packed structure. Atoms can be erased by long-pressing.

deleted the second layer
If it is a hexagonal close-packed structure, the layers are arranged like A-B-A-B-A-B….so the first and third layers are the same. Therefore, one atom in the first layer will cover up one of the atoms in the third layer.
In the case of the face-centered cubic lattice shown above, the layers are arranged like A-B-C-A-B-C-A-B-C…. The first and third layers are different. Therefore, one atom in the first layer does not completely overlap with any atom in the third layer.
Download ‘Crystal Lattice’

Other apps
N’Back 10
n-back app up to 10’back
N’Back 10 Dual
Dual task version of “N’Back 10”
GP Counter
A general purpose counter that records the timestamp of the count
Audio SA
Spectrum Analyzer app that can take voiceprint
2 Digit Multiplication
Learning 2-digit x 2-digit multiplication method that can be used for mental arithmetic
Fractal Catalog
A collection of images with fractal properties
Jigsaw Puzzle Maker 2, Jigsaw Puzzle Maker for iPad
Create a jigsaw puzzle from your favorite photos